menu
menu
Our mission
Orano Med seeks to develop a new generation of targeted therapies against cancer using the unique properties of lead-212 (212Pb), a rare alpha-emitting radioisotope and one of the most potent therapeutic payloads against cancer cells.
Although oncology research has made tremendous progress in recent years, medical needs remain unmet for many types of cancer. Our approach, known as Targeted Alpha Therapy (TAT), opens up promising prospects for patients not responding
to existing treatments.
Our strategy
Making successful Targeted Alpha Therapy a reality
Orano Med’s strategy has two goals:
- developing a robust pipeline of 212Pb-based therapies,
- providing a reliable supply chain for these innovative drugs.
Orano Med masters 212Pb chemistry and conjugation technology (including proprietary antibody site specific conjugation via its subsidiary Macrocyclics acquired in 2011) and is able to radiolabel 212Pb to various biological vectors (peptides, antibodies, etc.) targeting antigens or receptors expressed in a wide range of cancers.

Develop a pipeline of 212Pb-based anti-cancer treatments
We focus our developments on areas of high unmet needs where 212Pb-labeled compounds could make a difference and, since 2016, we have been equipped with a preclinical facility to accelerate the transition of our molecules into clinical research.

Produce and ensure the availability of drugs
Because reliability of supply of alpha emitters has long hindered the development of TAT, we are also investing in our production facilities. Our facilities currently have the capacity to supply all the 212Pb necessary to conduct clinical trials and in the future will be able to ramp up to industrial production capacity to meet commercial needs.
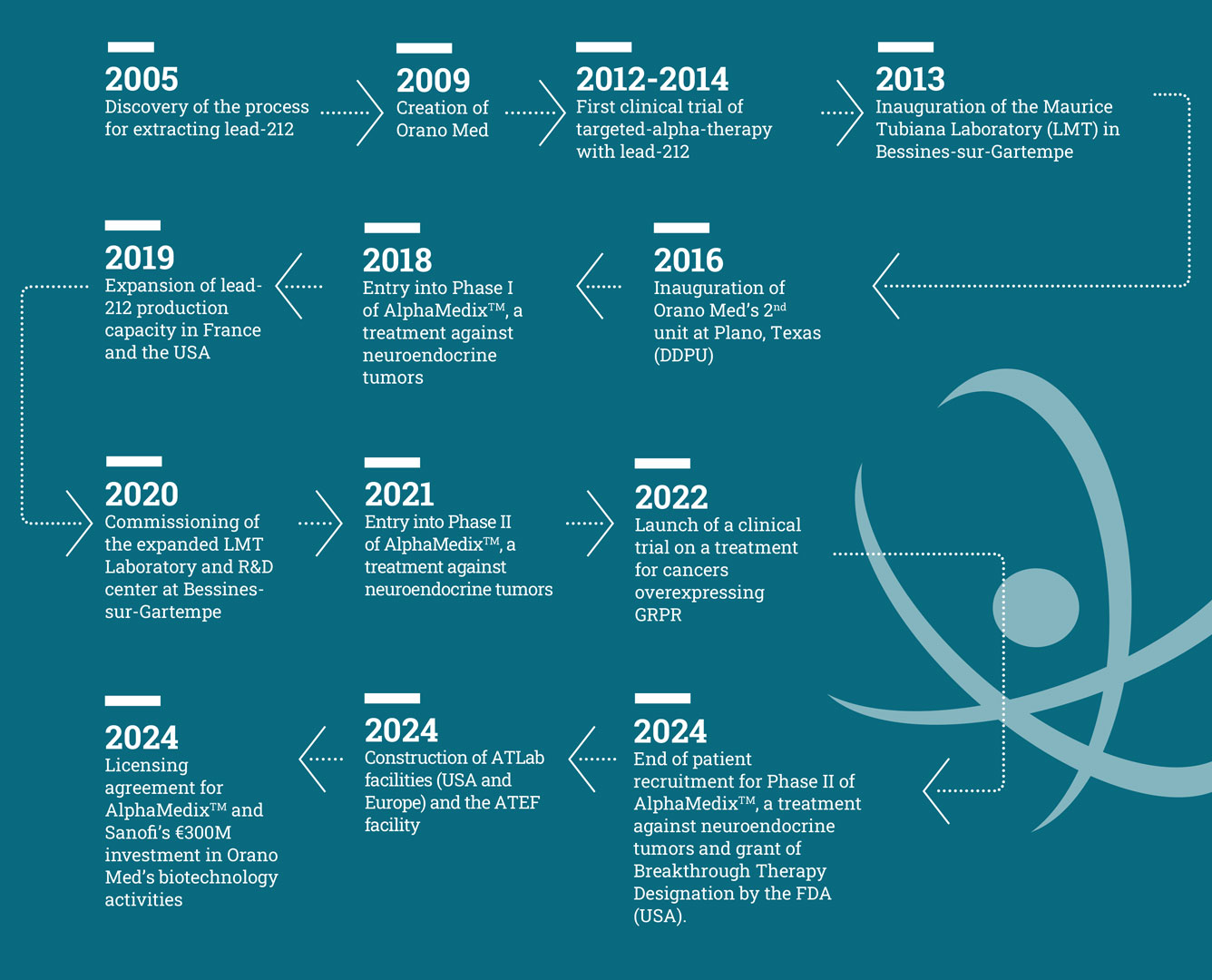
Our history
In the early 2000s, Areva (now Orano) looked at opportunities to use material derived from its core nuclear energy activities. Nuclear medicine and Radioligand Therapy quickly appeared very promising. 212Pb was identified as offering tangible scientific prospects, and Orano had the necessary know-how to meet the challenges involved in its procurement.
With sufficient resources to produce 212Pb in larger quantities, the project soon emerged to demonstrate the feasibility of extracting and purifying this isotope. Successful results followed by conclusive preclinical studies with the National Cancer Institute in the US led to the creation of Orano Med in 2009.